COVID-19 Clinical Trials Market Size Worth $9.9 Billion By 2027: Grand View Research, Inc.
The global COVID-19 clinical trials market size is expected to reach USD 9.9 billion by 2027, registering a CAGR of 9.5% during the forecast period, according to a new report by Grand View Research, Inc. An increasing number of deaths due to coronavirus is creating a need to develop effective treatments, thereby boosting the market growth. Also, the underlying economic profits for first movers are encouraging pharmaceutical players to invest in clinical trial studies for COVID-19.
The current pandemic poses an acute health risk to the entire population. A key to successfully fighting COVID-19 lies in clinical research. At present, almost all the major research-based pharmaceutical companies, many other biotechnology and pharmaceutical companies, as well as research institutes are engaged in a race to develop an effective treatment against coronavirus. There are currently 661 unique compounds in development against COVID-19, of which 292 are drugs, 173 are vaccines and 196 are antivirals.
Request a sample Copy of the Global COVID-19 Clinical Trials Market Research Report @ https://www.grandviewresearch.com/industry-analysis/covid-19-clinical-trials-market/request/rs1
The regulators in the U.S. and Europe also offer various options for action and procedural facilitation to enable faster access to effective vaccines and drugs to combat the pandemic. In March 2020, the Solidarity Trial-an international clinical trial by the World Health Organization (WHO)-was launched to find effective treatment against COVID-19. In May 2020, WHO also announced an international alliance for developing various candidate vaccines to stop the spread of coronavirus disease, calling this effort the Solidarity trial for vaccines.
The current scenario across the globe and the need to come up with treatment options has also led to the fast-track of clinical trials. Hence, in March 2020, the Food and Drug Administration (FDA) launched a Coronavirus Treatment Acceleration Program (CTAP) for the likely therapies to accelerate the development of the treatment for the pandemic caused by a coronavirus. Recently in May 2020, the U.S. FDA granted fast track title to Moderna’s mRNA vaccine candidate, mRNA-1273, to protect against COVID-19.
Key suggestions from the report:
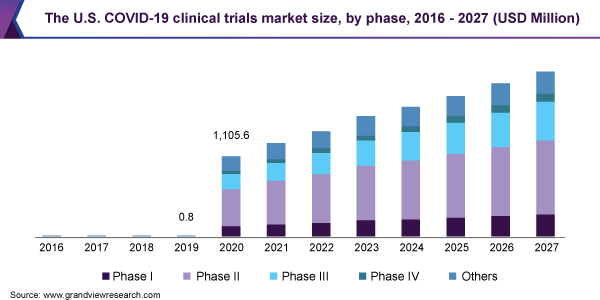
- Phase II COVID-19 clinical trials dominated the market with a share of 35.0% in 2020. This is attributed to the maximum number of products currently in development.
- Vaccines segment accounted for the largest share of 77.1% in 2020 owing to the increasing investments towards it.
- Europe held 46.8% of the market share in 2020. Favorable government initiatives such as the UK’s “ACCORD” clinical trial program are contributing to market growth.
- The Asia Pacific region is projected to witness the fastest CAGR of 11.8% during the forecast period as the increasing number of biotechnology firms are looking at the region for their COVID-19 trials to take advantage of the large patient pool and fast-track procedures.

Have Any Query? Ask Our Experts @ https://www.grandviewresearch.com/inquiry/450691/ibb
Grand View Research has segmented the global COVID-19 clinical trials market based on phase, product, and region:
COVID-19 Clinical Trials Market Phase Outlook (Revenue, USD Million, 2016 – 2027)
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
COVID-19 Clinical Trials Market Product Outlook (Revenue, USD Million, 2016 – 2027)
- Therapeutics
- Vaccines
COVID-19 Clinical Trials Market Regional Outlook (Revenue, USD Million, 2016 – 2027)
- North America
- The U.S.
- Canada
- Europe
- The U.K.
- Germany
- France
- Spain
- Italy
- Asia Pacific
- India
- Japan
- China
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- The Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
List of Key Players of COVID-19 Clinical Trials Market
- Moderna, Inc.
- GlaxoSmithKline plc
- Pfizer Inc.
- Johnson & Johnson
- Gilead Sciences Inc.
- INOVIO Pharmaceuticals
- AbbVie Inc.
- BioNTech SE
- Novavax
- Takeda
Find more research reports on Medical Devices Industry, by Grand View Research:
Vascular Imaging Market – The global vascular imaging market size was valued at USD 17.5 billion in 2018 and is projected to expand at a CAGR of 4.2% by 2026.
Advanced Ophthalmology Technologies Market – The global advanced ophthalmology technologies market size was valued at USD 5.8 billion in 2018 and is projected to witness a CAGR of 6.5% by 2026.
About Grand View Research
Grand View Research provides syndicated as well as customized research reports and consulting services on 46 industries across 25 major countries worldwide. This U.S.-based market research and consulting company is registered in California and headquartered in San Francisco. Comprising over 425 analysts and consultants, the company adds 1200+ market research reports to its extensive database each year. Supported by an interactive market intelligence platform, the team at Grand View Research guides Fortune 500 companies and prominent academic institutes in comprehending the global and regional business environment and carefully identifying future opportunities.
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:201, Spear Street, 1100
City: San Francisco
State: California
Country: United States
Website: https://www.grandviewresearch.com/industry-analysis/covid-19-clinical-trials-market